Isolation & Crystallization
Thermodynamic Background
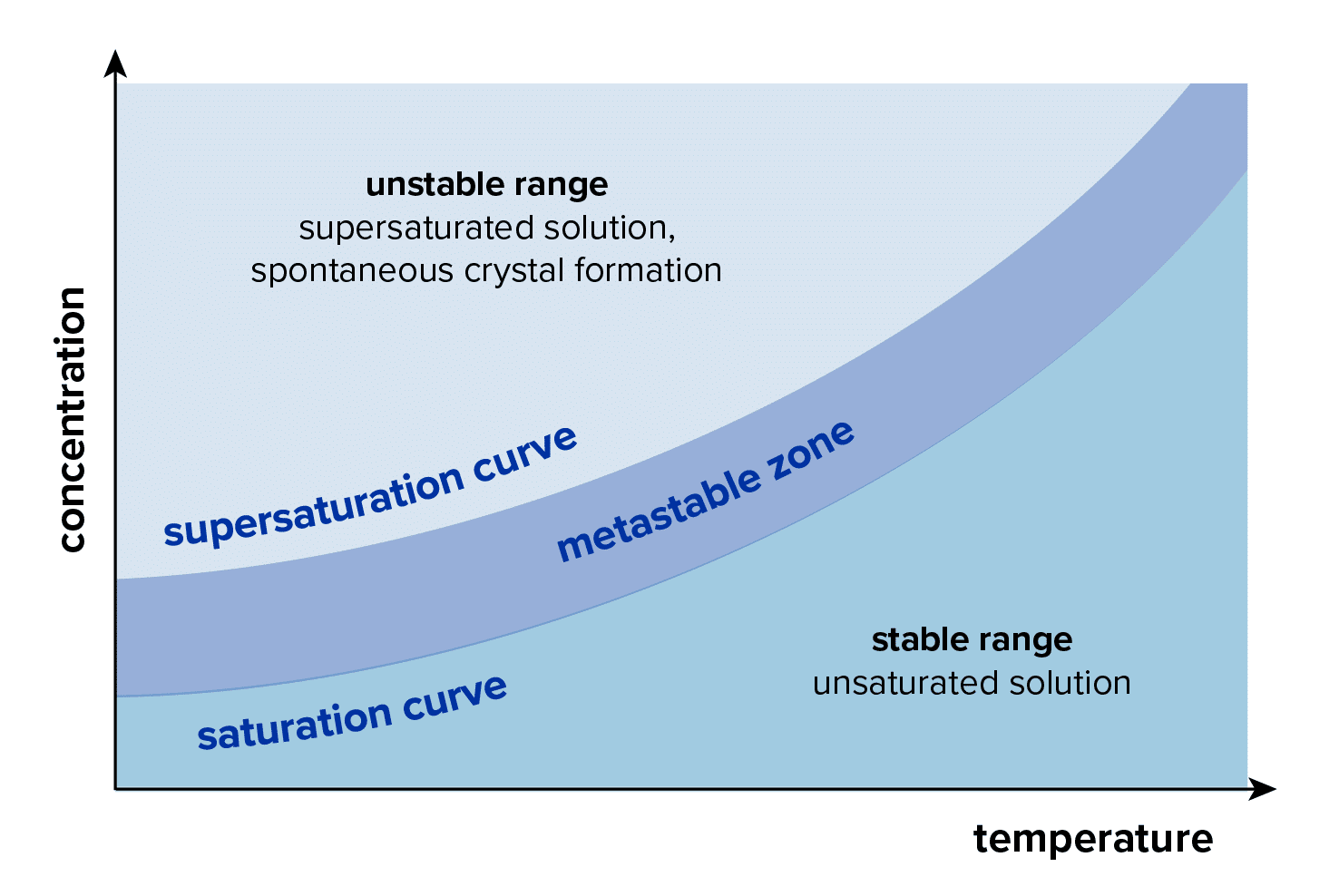
Crystallization can occur due to the limited solubility of materials in a solvent. Many factors come into play, but ultimately under the right conditions many organic compounds will begin to form organized lattices, or crystals, which are a lower energy state than when dissolved in a supersaturated solution.
By controlling the various factors of the process, the crystals can form with very high concentrations of the target molecule, excluding any impurities. The ability for an organic substance to crystallize is dependent on intramolecular and intermolecular forces. For certain compounds, the result of the solidification may instead form an amorphous solid.
Cooling Crystallization
Cooling crystallization takes advantage of the fact that many organic compounds have reduced solubility with reduced temperature. Pope’s recommendation is to use a jacketed reactor paired with a temperature control unit to bring the temperature of the solution down. Eventually, the solution will become supersaturated. The formation of crystals in the supersaturated solution is dependent on many factors including any impurities that may be present, the mixing profile, and whether seed crystals are utilized.
To ensure the saturation limits of the solution are not altered significantly it is important to purify the starting material as much as possible prior to crystallization (by means such as distillation). With varying impurities, the temperatures at which a material crystallizes as well as the overall purity and yield can vary greatly.
Mixing profile in the crystallizing vessel is also very important as this can have an impact on the nucleation (formation) of crystals vs the growth stage for crystals. Depending on the ultimate use of your crystalline product, certain particle sizes and particle size distributions may be beneficial.
The use of seed crystals can also be vital as this can impact the quantity and size of crystals formed. Seed crystals can also be critical in preventing the formation of amorphous solids.
Isolation on a Nutsche Filter Dryer
Nutsche filter dryers are valuable tools in the GMP environment. After a crystal slurry is created the Nutsche filter dryer can be used as a single container for the multiple unit operations that are required.
First the slurry is transferred to the Nutsche where the “mother liquor” or supernantant can be separated from the crystalline product using a fine filter screen. After the material is filtered, a pure solvent is typically added to remove any impurities that may be present on the surface of the crystals, often called a “wash” step. The washed crystals at this point should be of a high purity. These crystals can then be dried by applying heat and vacuum to the Nutsche filter dryer. The agitator in the Nutsche allows for the cake to be smoothed which results in even drying and a product should be considered solvent free.

